MICHAEL GIER
Sales Pharma & Healthcare
Phone +49 4442 982-3961
As an experienced manufacturer of pharmaceutical and medical plastic packaging, we offer customized solutions for primary and secondary packaging in highly sensitive applications. Our products meet the most stringent requirements for cleanliness, hygiene, and quality—from GMP-compliant injection-molded packaging to ISO 13485-certified system solutions.
We develop and produce plastic syringe packaging, tablet packaging, and nasal spray atomizer components using validated processes. Complex packaging solutions such as nests and tubs are created in our modern cleanroom production facility – fully automated and low in particles.
As a contract manufacturer in the pharmaceutical sector, we also implement packaging solutions according to your specifications. We combine our many years of experience with innovative manufacturing technology to ensure maximum product safety and efficiency. Our expertise makes us a reliable partner for pharmaceutical packaging and medical devices when it comes to precision, GMP compliance, and uncompromising quality.

GMP-compliant pharmaceutical syringe packaging for glass and plastic syringes

with screw cap – ideal for pharmacy supplies and pharmaceutical applications
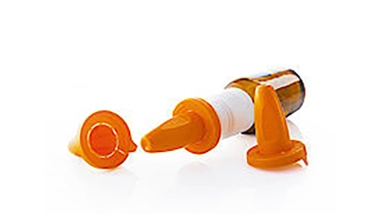
for nasal spray atomizers – manufactured with the highest precision for safe medication dispensing
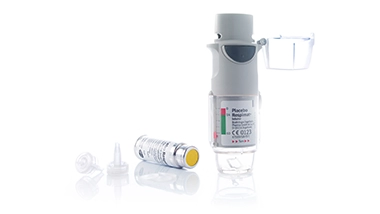
with individual components – manufactured under GMP conditions

for throat lozenge containers – secure protection and convenient opening

with spring segment stopper – ideal as tablet packaging in the healthcare sector

with flip lid – robust and secure secondary packaging

and screw caps for medicine bottles and liquid preparations
Using state-of-the-art injection molding machines, precision assembly technology, and our own toolmaking facilities, we develop and produce pharmaceutical plastic packaging and medical packaging solutions tailored to your requirements. From prototype development and validation to series production, we are your reliable partner – even for complex projects, innovative new developments, and sensitive pharmaceutical packaging. Our expertise covers primary packaging such as syringe packaging, tablet packaging, and nasal spray atomizer components, as well as secondary packaging and complete system solutions.
Our GMP-compliant injection molding production in a fully automated cleanroom enables us to provide a low-particle production environment – ideal for highly sensitive applications in the pharmaceutical and medical sectors. We efficiently, economically, and flexibly produce packaging that meets the highest standards of quality, safety,
We will gladly review your specific application case.

Sales Pharma & Healthcare
Phone +49 4442 982-3961
We would be happy to present our advantages to you in a personal meeting.
From the start of component development to series production, the highest demands are placed on technology and quality. The manufacturing and documentation of our products are carried out in accordance with the rules of Good Manufacturing Practice (GMP) and DIN EN ISO 13485.